Introduction: Mannan-binding lectin-associated serine protease-3 (MASP-3) activates pro-complement Factor D (CFD) to form mature CFD, a rate-limiting enzyme in the alternative pathway (AP) of the complement system. CFD controls cleavage of complement Factor B (CFB) to form AP C3 convertase, which drives an amplification loop of protease complexes that activates both C3b-mediated opsonization and the terminal pathway. OMS906 is a humanized IgG4 monoclonal antibody (mAb) that binds to and inhibits MASP-3, thereby blocking maturation of CFD and downstream AP activity. Data from mice and monkeys demonstrated that OMS906 is highly selective and effectively inhibits AP activity. MASP-3 inhibition could provide therapeutic benefit in a variety of AP-mediated diseases, such as paroxysmal nocturnal hemoglobinuria (PNH). In PNH, a rare and life-threatening disorder, red blood cells (RBCs) lacking CD59 and CD55 regulatory surface proteins are targeted for rapid clearance via intravascular hemolysis and extravascular hemolysis, due to AP dysregulation. Clinical efficacy of OMS906 in a study of patients with PNH has been reported. To provide mechanistic support, we conducted in vitro and in vivo studies using preclinical models of complement-mediated hemolysis that have mechanistic overlap with PNH.
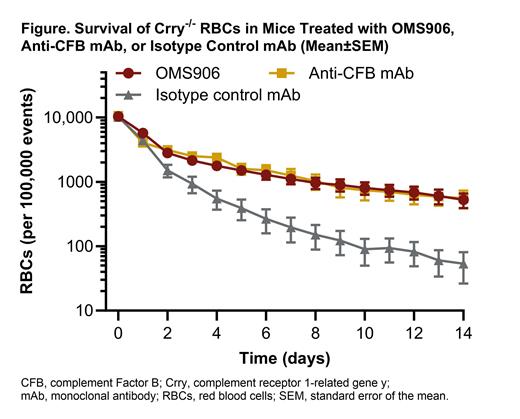
Methods:In vitro potency of OMS906 was assessed using RBCs deficient in CD55 and CD59 to mimic the physiological effect of PNH. Healthy human donor RBCs were treated with inhibitory CD55 and CD59 antibodies, then incubated under AP assay conditions with human serum (diluted to 50%) containing OMS906, anti-C5 IgG4 mAb, or an isotype mAb control. The potency of OMS906, expressed as the half-maximal inhibitory concentration (IC 50), was assessed based on prevention of hemolysis of the PNH-like RBCs in vitro. Lysis was quantified by measuring hemoglobin released into sample supernatants using spectrophotometric absorbance. The in vivo effect of OMS906 on survival of murine RBCs prone to rapid clearance was assessed using RBCs from Crry -/- mice, which lack a rodent-specific complement regulatory protein that blocks AP activation. C57BL/6J male mice were intravenously injected with fluorescent labelled Crry -/- RBCs and received OMS906 or isotype mAb control via subcutaneous injection, or anti-CFB mAb via intraperitoneal injection. Blood samples were taken daily until Day 14 and the remaining number of Crry -/- RBCs measured by flow cytometry.
Results:In vitro, OMS906 inhibited terminal cell lysis in human PNH-like RBCs more potently than a C5 inhibitor. Comparison of representative drug concentration-response showed that the average functional potency was 58-fold greater with OMS906 (IC 50: 0.8 μg/mL; 3 experiments; 12 total RBC donors) than with anti-C5 mAb (IC 50: 46.2 μg/mL; 2 experiments; 4 total RBC donors). In addition, the maximal extent to which lysis was inhibited was consistently greater with OMS906 than with anti-C5 mAb. In vivo, the rate of decline in number of Crry -/- RBCs was significantly slowed in mice treated with OMS906, in contrast to the rapid loss observed in isotype mAb control-treated mice ( Figure). OMS906 recipients showed a rate of Crry -/- RBC survival similar to the anti-CFB mAb group.
Conclusion: OMS906 inhibited RBC lysis more potently than a C5 inhibitor, providing evidence that MASP-3 inhibition blocks downstream terminal activity and thus intravascular hemolysis. In addition, OMS906 improved survival of Crry -/- RBCs comparably to a CFB inhibitor, indicating that MASP-3 inhibition prevents the AP-mediated destruction of RBCs predictive of extravascular hemolysis. OMS906 is currently in clinical development for the treatment of PNH.
Disclosures
Li:Omeros Corporation: Current Employment, Current equity holder in publicly-traded company. Yabuki:Omeros Corporation: Current Employment, Current equity holder in publicly-traded company. Cummings:Omeros Corporation: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal